OnPoint Vision Clinical Study
An FDA investigational treatment to improve NEAR vision in patients
with late-stage AMD
OnPoint Vision Clinical Study
An FDA investigational treatment to improve NEAR vision in patients
with moderate to late-stage AMD
The FDA Study & The Technology
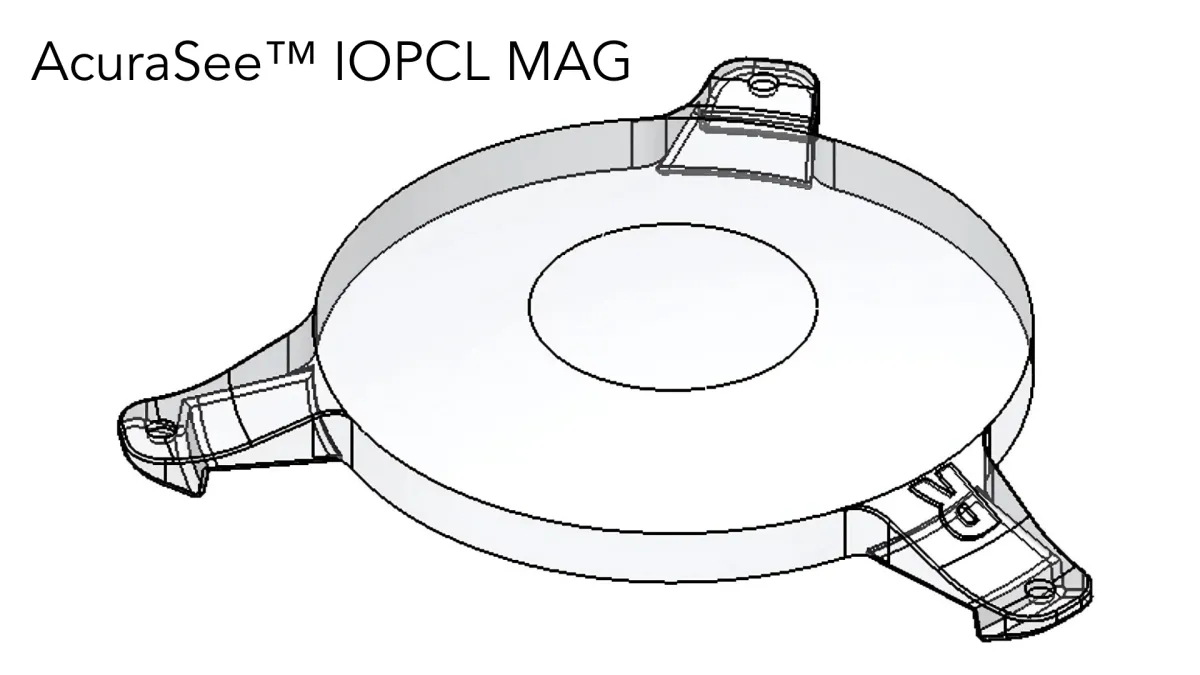
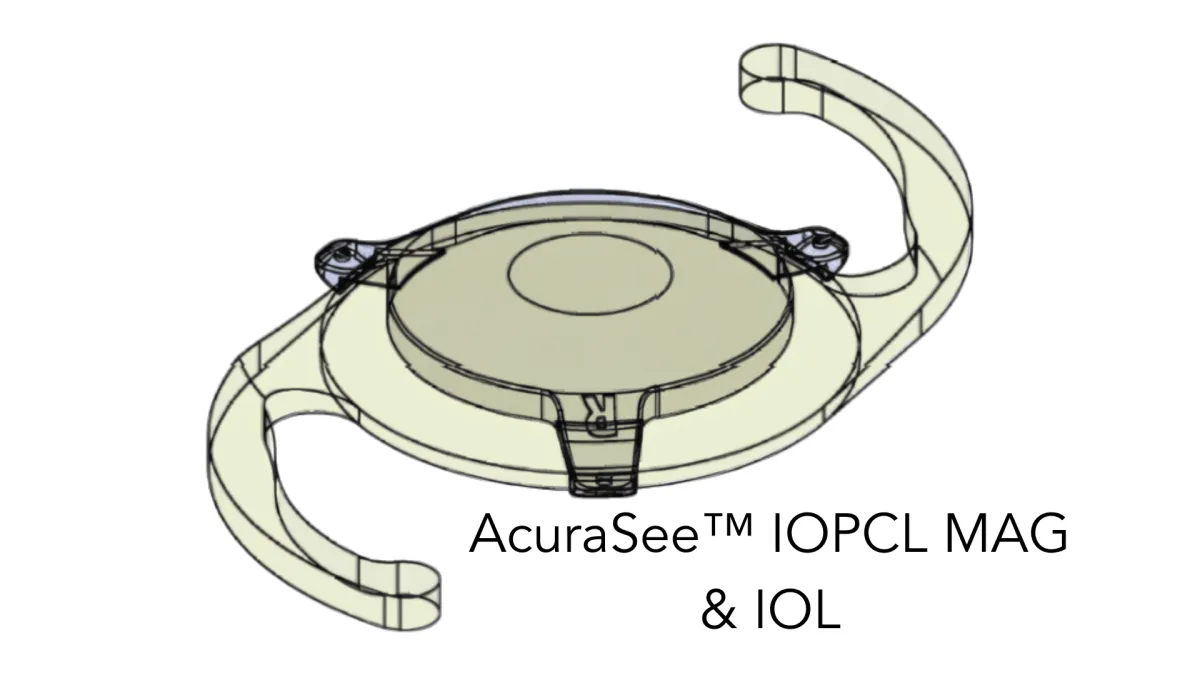
The IOPCL (Intra-Ocular Pseudophakic Capsular Lens) known as the AccuraSee® shares the space in the capsular bag, coupled to the anterior surface of an existing 6mm acrylic monofocal IOL (see images below).
The IOPCL has no power with the exception of the central portion which has a 1.8mm 10.0D magnified zone which translates to 7.0D at the spectacle plane. The IOPCL has no impact on distance or intermediate vision. The IOPCL is implanted in the non-dominant eye. In our Early Feasibility Study subjects with a BCDVA between 20/80 and 20/250 gained an average of 5 lines of improvement in UCNVA from baseline.

Become a Retina Sub-Investigator
We invite retina specialists who are interested in learning more about the study to complete the form. A 15-minute call will be scheduled with our Head of Clinical Affairs to discuss the study in detail.
Become a Retina
Sub-Investigator
We invite interested retina surgeons to complete the form below to schedule a 15-minute informational call with our Director of Clinical Research. During this call, we will review the role of serving as a sub investigator by sending eligible candidates to a study cataract surgeon. Which will allow eligible patients to be considered for participation in the study at no cost. This call will also include a review of the study inclusion criteria and identification of the study cataract site closest to your practice.
The following questions are intended to better understand your background and interest in this clinical study:
Candidates for The OnPoint Vision Clinical Study
Must have AMD in both eyes + Cataract surgery at least 6 months ago
55 years or older.
Be able to make an informed decision
Does not drive at night.

There is no cost to the patient for participating in the clinical study.

